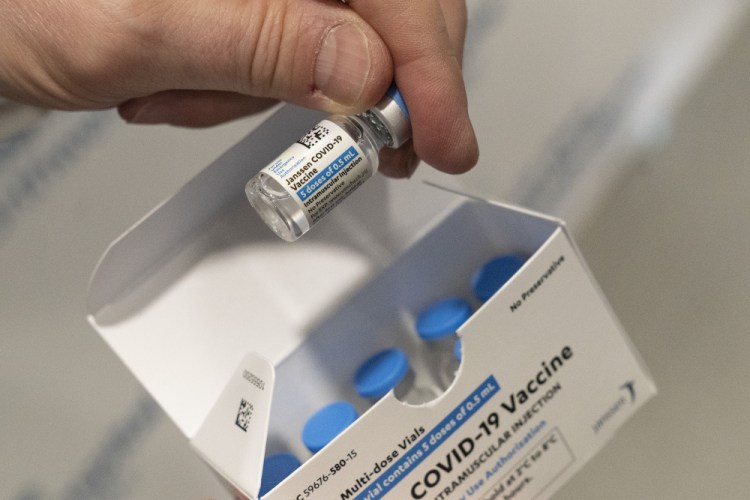
The Baltimore factory hired to help make Johnson & Johnson’s COVID-19 vaccine was dirty, didn’t follow proper manufacturing procedures and had poorly trained staff, resulting in contamination of material going into a batch of shots, U.S. regulators said Wednesday.
The Food and Drug Administration released a statement and a 13-page report detailing findings from its just-completed inspection of the idled Emergent BioSciences factory.
Agency inspectors said a batch of bulk drug substance for J&J’s single-shot vaccine was contaminated with material used to make COVID-19 vaccines for another Emergent client, AstraZeneca. The batch, reportedly enough to make about 15 million J&J vaccine doses, had to be thrown out.
Other problems cited in the inspection report included peeling paint, black and brown residue on factory floors and walls, inadequate cleaning and employees not following procedures to prevent contamination between vaccine batches and ingredients.
Nothing made at the factory for J&J has been distributed, the FDA noted. The nearly 8 million doses of J&J vaccine given in the U.S. came from Europe.
Both Emergent and Johnson & Johnson said Wednesday that they are working to fix the problems as quickly as possible.
After quality problems surfaced late last month, J&J took control of the factory. The Biden administration now is working to move AstraZeneca vaccine manufacturing to another factory. AstraZeneca has yet to seek emergency authorization for use of its vaccine in the U.S.
The Baltimore factory halted all production late last week at the FDA’s request. The agency hasn’t given emergency approval to the factory, which is needed before any vaccine material made there can be distributed.
All the bulk vaccine substance inside Emergent’s factory, plus early batches made there and then put in vials and packaged by other J&J contractors, are being stored and will undergo additional testing by the FDA, the agency said.
“We are doing everything we can to ensure that the COVID-19 vaccines that are given to the people of this nation have met the agency’s high standards for quality, safety and effectiveness,” the FDA said.
At the moment, use of the J&J vaccine is on hold in the U.S. as government health officials investigate its possible connection to rare blood clots. Their decision on whether to allow its use to resume could come Friday.
On Tuesday, the European Medicines Agency’s safety committee concluded the blood clots are a very rare side effect but that the vaccine’s benefits outweigh that risk.
Emergent, a little-known drug manufacturing contractor, was granted a major role in the Trump administration’s response to the coronavirus. The company has been repeatedly cited by the FDA for problems ranging from poorly trained employees to cracked vials and mold around one of its facilities, according to records obtained by The Associated Press.
After the J&J vaccine batch was contaminated, FDA inspectors started checking the Emergent factory on April 12 and finished their investigation on Tuesday.
The inspectors also reviewed security camera footage, which showed employees carrying unsealed bags of medical waste around in the factory, with the bags touching materials ready to be used to make vaccine batches.
The footage also showed employees moving between manufacturing areas for the two vaccines without documenting whether they changed protective gowns and showered in between, as well as removing protective clothing in the warehouse near prepared vaccine ingredients, dropping the clothing on the floor and then tossing it in open trash cans.
The report noted that Emergent didn’t sufficiently investigate the contamination of the later-discarded J&J batch and didn’t appear to have done any extra cleaning after it was discovered.
“There is no assurance that other batches have not been subject to cross-contamination,” the report stated.
It noted other problems at the factory, known as Bayview: inadequate procedures for assuring the vaccine met all quality and purity requirements, and floors and walls too damaged to be properly sanitized. The plant also was too small and not properly designed for thorough cleaning, maintenance and operations, inspectors said.
It’s unclear how long it will take the companies to resolve all the problems.
J&J has pledged to provide 100 million doses for the U.S. by the end of May and 1 billion doses globally by the end of the year.
“Right now, we can’t speculate on any potential impact this could have on the timing of our vaccine deliveries,” J&J said in a statement.
Send questions/comments to the editors.

Comments are no longer available on this story